CHAPTER 6
EARLY HARVEST: THE UPPER ATMOSPHERE AND COSMIC RAYS
Scheduling V-2 flights, developing newer rockets, testing instruments, seeking financial support, fighting military classification, arguing and politicking in meetings national and international-such activities seemed to consume more time and energy than the actual science that was their ultimate purpose. But because of those subsidiary activities, which fill most of the pages of this book, the scientific research moved steadily forward. Month by month, year by year the results accumulated. By the time NASA began to operate, a rich harvest had already been reaped from sounding rockets, with several significant contributions from the scientific satellite program of the International Geophysical Year. These, especially upper atmospheric and cosmic ray research, gave NASA a running start in space science.
By the early 1960s the study of energetic particles and magnetic fields from the sun and their interaction with the earth’s magnetic field had become a well integrated and coherent field of study. By then, also, satellite geodesy had begun to make its mark. But the space science program was open ended, and the harvest a continuing one. This steady advance of space science is the subject of three chapters (6, 11, 20), whose aim is to present in broad outline what the space science disciplines encompassed and to show how space techniques made notable contributions. The present chapter reviews achievements through 1958.
THE THRESHOLD TO SPACE
Thirty thousand light-years from the center of a disk-shaped galaxy, itself measuring 100 000 light-years from edge to edge, planet Earth revolves endlessly around an average star, the sun, which with its attendant planets speeds toward remote Vega, brightest star in the northern skies. Although containing billions of stars, nebulas, and other celestial objects, most of the galaxy consists of empty, or nearly empty, space. To inhabitants of Earth the threshold to these outer voids is the upper atmosphere.
One can easily show theoretically that the pressure and density of the atmosphere must decrease exponentially with increasing height above the ground, and experiment confirms this conclusion.1 Roughly, at least for the first hundred kilometers, pressure and density fall to one-tenth their former value for every 10-mile (16-km) increase in altitude. Hence, above 30 km only one percent of the atmosphere remains, while beyond 100 km lies only one-millionth of the atmosphere.
Interest in the lower atmosphere where people live and experience the continuous round of changes in weather and climate is obvious, but one might well ask what could possibly hold the attention, even of scientists, in a region so nearly empty as the upper atmosphere? The initial impression, however, is misleading. After closer study the upper atmosphere is found to exhibit many fascinating, often practically important phenomena-such as the ionosphere, which profoundly influences radio communications, especially shortwave; the auroras; electric currents, which at times cause magnetic effects that blank out both radio and telephone links; and the ozonosphere, which during the debate over fluorocarbon-propelled aerosols gained temporary stature in the public mind as the protecting layer that shields the earth’s surface from life-killing ultraviolet rays of the sun. So interesting were the challenging phenomena of the upper atmosphere that by the time sounding rockets put in their appearance, scientists had already evolved from afar a coherent, comprehensive picture of the upper atmosphere and solar-terrestrial relationships. In the mid-1940s this remarkably complete picture formed a paradigm that hundreds of geophysicists around the world shared and used in reporting their continuing researches at scientific meetings and in the literature.
The main features of this paradigm were set forth in an article on the upper atmosphere by B. Haurwitz, first published in 1936 and 1937 and reissued with some updating in 1941.2 For those who began using sounding rockets in 1946 to explore the upper atmosphere, Haurwitz’s concise review provided a helpful introduction, while a review paper by T. H. Johnson told much of what was known about cosmic rays from ground-based and balloon researches.3 A classic paper by Fred Whipple on the use of meteor observations to deduce atmospheric densities at altitudes between 50 and 110 km was one of the best examples of the ingenuity necessary in studying a region not yet accessible to them or their instruments.4 But the work that best described the state of knowledge of the earth’s high atmosphere at the very time when the sounding rocket program was getting under way in the United States was a book of more than 600 pages, The Upper Atmosphere, by Indian scientist S. K. Mitra. Mitra furnished an exhaustive review of upper-atmospheric research, concluding with a chapter summarizing what had been learned and listing some of the most important problems needing further research. The very last paragraph noted that as the volume was going to press word had reached him:
that experiments are being conducted in the U.S.A. with the V-2 rockets to study the cosmic rays and the ionospheric conductivity up to heights of 150 km (August, 1946). It is hoped that the scope of these experiments will be extended and that the records obtained therefrom will, on the one hand, give direct information of upper atmospheric conditions and on the other reveal the true picture of the intensity distribution of solar ultraviolet radiation and thus help to solve the many mysteries of the upper atmosphere which till now have resisted all attacks.5
Mitra’s hopes paralleled the motivations of the sounding rocket experimenters, many of whom were entering what was to them an entirely new field.
Following is an elaboration of the extensive paradigm that the space scientists inherited from the ground-based researchers (fig. 1). The description is based on the works cited above, especially on Mitra’s treatise.
The atmosphere extended to great heights, auroras being observed on occasion to more than 1000 km. Pressure and density were calculated and observed to fall off in exponential fashion. If the temperature were uniform throughout the atmosphere, the decline in these quantities would be given by
\[ p = p_0 \exp(\frac{-h}{H}) \]and
\[ \rho = \rho_0 \exp(\frac{-h}{H}) \]where p and p denoted pressure and density respectively, the subscript zero indicated values at the ground, and h was altitude. The quantity H, known as the scale height, was given by
\[ H = kT / Mg \]where
k = Boltzmann constant = 1.372X 10-16 erg/degree
T = temperature in kelvins
M = mean molecular mass of the air = 4.8 X 10-23 gm
g = acceleration of gravity.6
In (1) and (2) the value of g was assumed to be constant, whereas in actuality gravity varies inversely as the square of the distance from the center of the earth. Hence the expressions for pressure and density were only approximate. More significantly, atmospheric temperature varied markedly with altitude, the scale height given by (3) varying proportionately. Thus, in regions of high temperature the pressure and density declined more slowly with height than where the temperature was low.
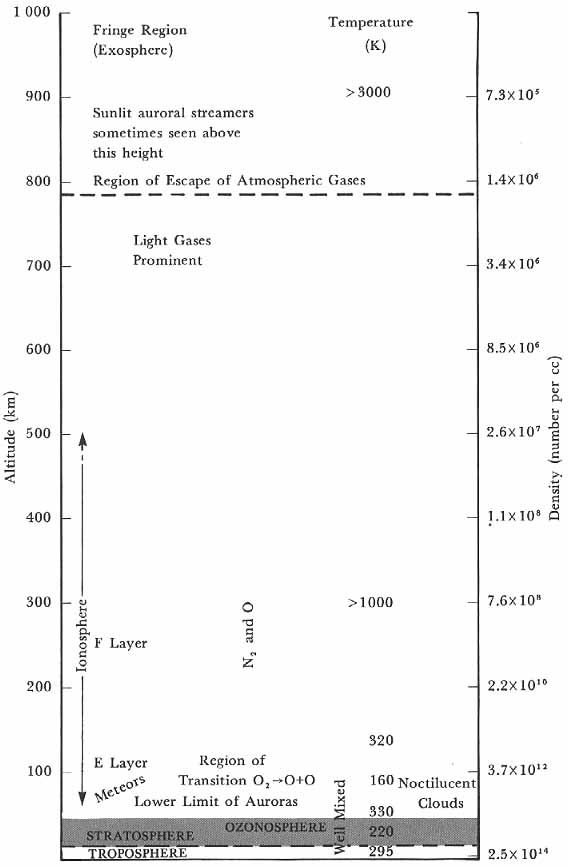
The upper atmosphere as visualized in the mid-1940s.
In regions where the atmospheric temperature was constant or nearly so, each separate atmospheric gas individually followed laws like (1) and (2) with the average molecular mass M in (3) replaced by the molecular mass of the individual gas. Thus, the corresponding scale height H varied inversely as the molecular mass of the gas, and a heavy gas like carbon dioxide fell off in density much more rapidly than nitrogen, oxygen slightly faster than nitrogen, and light gases like helium much more slowly. As a result the lighter gases appeared to diffuse upward, while the heavier gases settled out. Such considerations led one to suppose that at the highest altitudes, hundreds or thousands of kilometers above the ground, the lighter gases predominated in the atmosphere. The outermost regions were expected to consist of hydrogen or helium primarily, although no experimental evidence confirmed the supposition.
Starting at the ground, atmospheric temperature fell at a rate of about 6 K per km throughout the troposphere (or “weathersphere") to a value of around 220 K at the tropopause, or top of the troposphere, which was found at 10 to 14 km, the lower height corresponding to higher latitudes, the greater height to the tropics. Above the tropopause the temperature remained fairly constant to about 35 km. A slight increase in the proportion of helium in the air above 20 km suggested some tendency toward diffusive separation, which at one time led researchers to expect that the region above the tropopause would exhibit a layered structure-hence the name stratosphere for this quasi-isothermal region.
Above the stratosphere, temperature rose again, as shown by the fact that the sound from cannon fire and large explosions was reflected from these levels of the upper atmosphere. Observations on this anomalous propagation of sound waves permitted one to estimate that the air temperature was about 370 K at 55 km height. Noctilucent clouds between 70 and 90 km suggested a low temperature in the vicinity of 80 km. These extremely tenuous clouds were seen only in high latitudes and only when illuminated by the slanting rays from the sun below the horizon. With the assumption that the clouds were composed of ice crystals, the temperature around 80 km was estimated to be about 160 K. The study of meteors, investigation of the electrical properties of the high atmosphere by radio techniques, and observations of the auroras showed that temperatures rose again above 80 km to 300 K at 100 km, and to 1,000 K or possibly 1,500 K at 300 km, with much higher temperatures beyond. Calculations from auroral observations were, however, not always consistent with this picture, often indicating considerably lower temperatures than those deduced from radio observations. Atmospheric composition near the ground was known to be:
| (percentage by volume) | ||
|---|---|---|
| Nitrogen | 78.08 | 99.9 |
| Oxygen | 20.95 | |
| Argon | 0.93 | |
| Carbon dioxide | 0.03 | |
| Neon | 1.8 x 10-3 | |
| Helium | 5 x 10-4 | |
| Krypton | 1 x 10-4 | |
| Xenon | 1 x 10-5 | |
| Ozone | Variable, > 1 x 10-6 | |
| Radon (average near ground) | 6 x 10-18 | |
| Hydrogen | Doubtful, < 1 x 10-3 |
Meteorological processes kept the atmosphere mixed, maintaining this composition at least up to 20 km. Between 20 and 25 km, helium increased about 3 percent above the normal value, but winds and turbulence kept the atmosphere well mixed far above stratospheric heights, up to at least 80 km.7
In the absence of other agents, this stirring should have kept the composition fairly uniform throughout the mixing regions. But solar ultraviolet radiation in the region from 1925 to 1760 , absorbed in atmospheric oxygen above the stratosphere, gave rise to a chain of reactions leading to the formation of ozone. Simultaneously solar ultraviolet in the neighborhood of 2,550 decomposed atmospheric ozone. An equilibrium between the formation and destruction of ozone, combined with atmospheric motions, distributed the gas so that in temperate latitudes it showed a maximum absolute concentration at about 25 km height, and a maximum percentage concentration at about 35 km. Although never more than the equivalent of a few millimeters at normal temperature and pressure, the ozone layer shielded the ground from lethal ultraviolet rays from the sun. Ozone concentrations were observed to be higher in the polar regions than in the tropics, and tended to correlate with cyclonic weather patterns.
Above 80 km, solar ultraviolet dissociated molecular oxygen, the dissociation becoming fairly complete by about 130 km. Thus the region from 80 to 130 km appeared as one of transition from an atmosphere consisting of mostly molecular nitrogen and molecular oxygen, to one of molecular nitrogen and atomic oxygen. It was assumed that above 100 km diffusive separation of the atmospheric gases became increasingly effective, and that the dissociation of oxygen enhanced the tendency of nitrogen to settle out and the oxygen to rise. Whether nitrogen also dissociated in the higher levels was not known.
In the upper levels of the atmosphere was the ionosphere. The term was used in two different ways, either to mean the ionized constituents of the high atmosphere, or to mean the regions in which the ionization was found.
An ionosphere was postulated by Balfour Stewart in 1878 to explain small daily variations observed in the earth’s magnetic field.8 Later, in 1902, A. E. Kennelly in America and O. Heaviside in England suggested that a conducting layer in the upper atmosphere, which could reflect radio waves beyond the horizon, might explain how Marconi in 1901 had sent wireless signals from Cornwall to Newfoundland.9 The first real evidence of such an ionosphere was obtained in 1925 when E. V. Appleton and M. Barnett in England detected sky waves coming down to their receiver after being reflected by a high-altitude layer.10 Additional evidence of the KennellyHeaviside layer came from experiments by G. Breit and M. A. Tuve in America.11 These experimenters sent a radio pulse upward, and observed two or more delayed pulses in a receiver a few kilometers away from the transmitter. The initial received pulse was assumed to be from the direct ray along the ground, and the other pulses to be echoes from the ionosphere. The method of Breit and Tuve became the basis for probing the ionosphere, using the reflections to determine the heights of various layers. Sophisticated formulas were worked out to explain how the various reflections observed were generated by the ionosphere. From these formulas and the experimental data, theorists could estimate layer heights, electron densities, magnetic field intensities, collision frequencies of the electrons and atmospheric particles, and reflection and absorption coefficients for the ionized media. 12
The ionization was assumed to be caused by solar radiations, and ultraviolet was taken to be the most likely agent. Sydney Chapman showed how a monochromatic beam of ultraviolet light would generate a parabolic distribution of electron concentrations in an exponential atmosphere of molecules (like oxygen) susceptible to ionization by the radiation (fig. 2).13 Starting with this basic theory and considering the effect of the various solar wavelength regions likely to influence the upper atmosphere, it was possible to estimate the variation with height of electron intensities and to make some guesses as to what the heavier ions might be.
From both radio observations and theory, scientists concluded that the ionosphere had two main regions of ionization, region E1 centering on 110 km, and region F2 centering on 275 km. The ionosphere was found to vary with time of day, season of the year, and phase of the sunspot cycle. For regions E1 and F2 halfway between the minimum and maximum of solar activity, the average ionization intensities corresponded to 105 and 106 electrons per cc, respectively.14 Mainly during the daytime, regions E2 and F1 formed at heights of 140 km and 200 km. Region D, at some uncertain distance below the E region, was observed at times of high solar activity, and presumably because of the increased molecular collision frequency at those lower altitudes caused pronounced absorption of radio signals of medium wavelength.
At great distances from the earth, the earth’s magnetic field was taken to be essentially that of a uniformly magnetized sphere; i.e., a magnetic dipole (fig. 3). Closer in, the field was observed to depart somewhat from that of a dipole, consisting of the dipole, or regular, part, and an irregular part. Some 94 percent of the earth’s field, including some of the irregular field, was found to have its origin inside the earth. Of the remaining 6 percent of external origin, about half appeared to be caused by a flow of electric current between the atmosphere and the earth. The remainder, about 3 percent of the total field, appeared to be due to overhead electric currents.15
Such electric currents could be produced by atmospheric motions at high altitude caused by solar or lunar tides, or by nonuniform heating of the atmosphere by the sun as the earth turned. While these more or less regular daily variations could easily be accounted for by electric currents in the ionosphere, magnetic storms which occurred at times of solar activity were more likely associated with streams of charged particles from the sun. The initial increase in magnetic field observed during a storm could be explained by the arrival of charged particles from the sun, which compressed the earth’s magnetic field slightly and thereby increased its value temporarily. The strong decline in intensity to below normal values which soon followed the initial phase might be caused by a huge ring current around the earth, fed by the particle stream from the sun, as suggested by Chapman, Ferraro, and others. Then the gradual recovery from this “main phase" of the magnetic storm, as it was called, signified the gradual dissipation of the ring current and a return to normal conditions-or so it was thought.
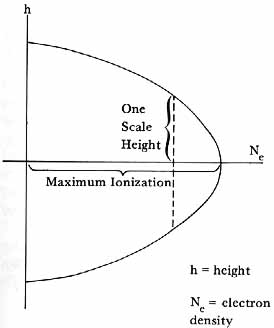
Chapman layer. The parabolic distribution was estimated to be within 5 percent of the actual distribution of charge densities to a distance of one scale height H ( = kT/mg) above and below the level of maximum ionization.
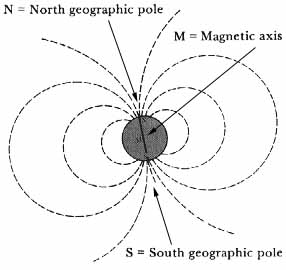
Earth’s magnetic field. The broken lines depict the lines parallel to the direction of magnetic force. As became increasingly clear over the years, the actual magnetic field of the earth differs considerably from this idealized picture of a dipole field.
Among the most notable of high-altitude phenomena, and among the earliest to be studied in detail, were the auroras, the northern and southern lights. These were seen most frequently at heights from 90 to 120 km, but also occurred at both lower and much greater heights. That the auroras correlated strongly with activity on the sun and appeared in an auroral belt at high latitude suggested that they must be due to charged particles from the sun. Charged particles would be steered by the earth’s magnetic field, whereas neutral particles or solar photons would not be affected by the earth’s field. Experimenting with cathode rays and small magnetized spheres, K. Birkeland in 1898 and others demonstrated how electrified particles approaching a magnetized sphere from a distance would be guided by the magnetic field toward the poles. Starting from Birkeland’s concepts and experiments, over many years Carl Stormer developed a theory of how electrons or protons from the sun would be deflected by the earth’s magnetic field into the auroral zones to produce the auroras as the particles impacted on the atmospheric molecules, causing them to glow.16 The spectrum of the aurora was observed to exhibit primarily lines and bands of atomic oxygen and molecular nitrogen, with the forbidden green lines of atomic oxygen at 5577 Å being particularly strong.
At nighttime the high atmosphere was seen to emit a very faint light, sometimes called the permanent aurora, also consisting of the forbidden lines of atomic oxygen and of bands of the nitrogen molecule. This air-glow was estimated to come from well above 100 km, perhaps from as high as 400 to 500 km, very likely from F-region ions as they were neutralized during the night. The yellow sodium D lines were also seen emanating from the lower part of the E region, and were particularly intense at twilight. From a distant cloud of material particles of some sort, the zodiacal light, with a spectrum similar to that of the sun, contributed to the light of the night sky. In the mid-1940s it was not known whether this radiation came from within the high atmosphere or from interplanetary space.
At some height, probably around 800 or 1,000 km, the atmosphere was expected to cease acting like a normal gas. In this region collisions between atmospheric particles would be infrequent, and a molecule might rise along an elliptic orbit to an apogee and fall back without colliding with another molecule until returning to the denser atmosphere at lower altitudes. If the molecule had sufficient velocity it might even escape into interplanetary space. Indeed, it was supposed that hydrogen and helium had to be escaping continuously through this fringe region, even though neither had been detected in the upper atmosphere. Helium was known to be entering the atmosphere from the ground-where it was produced by the decay of radioactive elements-at a small but measurable rate; but the percentage of helium in the lower atmosphere remained constant over time. The natural conclusion was that this light gas had to be diffusing up through the atmosphere to the highest levels where the very, high temperature permitted a ready escape of the gas.
Somewhere in this fringe region, or exosphere, the transition from the earth’s atmosphere to the medium of interplanetary space was assumed to lie. One was hard put to it to define the boundary. Presumably where the atmospheric density had dropped to the few particles per cubic centimeter expected in interplanetary space the boundary must already have been crossed. But long before then the atmosphere had ceased to exist in the usual sense of the term. Across this ill-defined interface, radiations from the sun entered the earth’s environs to cause the auroras, magnetic storms, ionization, and heating of the atmosphere.
Across this interface also came the cosmic rays.17 These highly energetic particles from outer space were more the concern of the high-energy physicist than of the geophysicist. Discovered between 1911 and 1914 from balloon experiments on atmospheric ionization, cosmic rays quickly became a subject of intense interest. It was soon accepted that the rays came from outside the earth. Measurements of the ionizing power of the rays at various depths below the surfaces of mountain lakes revealed both a soft component and a hard, or extremely penetrating, component to the rays. Balloon experiments showed that the intensity of the radiation increased steadily with altitude until a maximum-called the Pfotzer maximum-was reached at about 20 km in mid-latitudes. The shape of these intensity-altitude curves is shown in figure 4a.18 Figure 4b shows schematically that the earth’s magnetic field has a distinct effect upon the radiation, leading to the conclusion that the rays are charged particles, not photons.
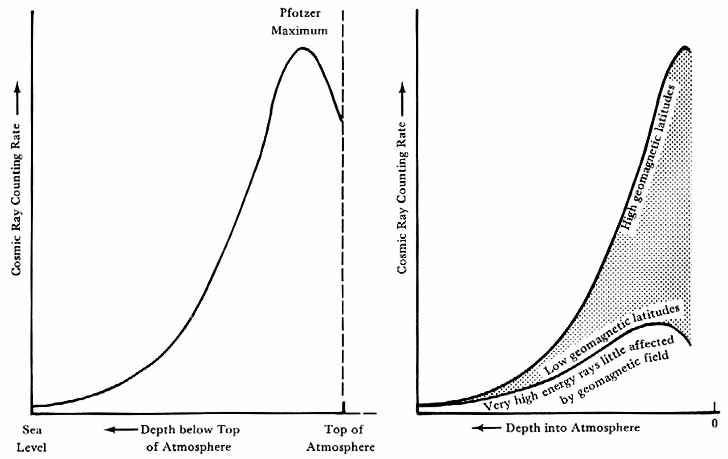
The shape of the intensity-altitude curve was explained as follows. The primary rays, whatever they might be, upon striking the atmosphere produced a shower of secondary rays, which, added to the primary rays, caused the initial increase in total ionization observed at high altitude. Eventually, however, an equilibrium was reached, with the atmosphere absorbing enough energy from both the primary and secondary particles to decrease the total ionizing power with further depth into the atmosphere. Such a transition curve, as it was called, would be observed not only in air, but also in lead or other substances, the principal difference being the spatial extent of the transitions, which depended on the density and nature of the material.
The early idea that the primary cosmic rays might be high-energy electrons was soon rejected. It could be shown that to penetrate the entire atmosphere and reach the ground, electron showers would have to be caused by primary electrons with such high energy that they would be completely unhindered by the earth’s magnetic field. They would accordingly not exhibit the magnetic field effect already shown to exist. In 1938 T. H. Johnson concluded that the primary radiation consisted of protons, as theorists had guessed somewhat earlier. In 1941 balloon observations revealed that the cosmic rays within the atmosphere at high altitude were mostly mesotrons (mesons), presumably generated by the primary protons.19 No significant component of electrons was observed at high altitude, supporting the conclusion that there could be no significant component of electrons in the primary radiation. But the soft component observed near the ground was believed to be electrons, decay products of the mesons generated at high altitude.
PROBLEMS TO SOLVE
Thus, the scientific paradigm for the earth’s upper atmosphere in the mid-1940s was rich in ideas accumulated over more than half a century of observation and theoretical study. It had been possible to explain to a considerable degree a wide range of phenomena, many of which proved to be extraordinarily complex; but many uncertainties, unanswered questions, and problems remained.
Consider the problem of estimating atmospheric densities in the E region of the ionosphere around 100-km altitude. In the 1920s F. A. Lindemann and G. M. B. Dobson approached this problem by using observational data on the heights of appearance and disappearance of visual meteors. Intuitively it seemed reasonable that the density of the gas traversed by a speeding meteor should play a role in determining where the meteor would glow and be visible. The challenge was to develop a suitable theory to relate the observed meteor trails to the atmospheric density. Lindemann and Dobson assumed that as the meteor rushed into the atmosphere, a hot gas cap formed because of compression of the air. Heat from the gas cap was transferred to the meteor, and if the object were small enough it became incandescent. Making a number of assumptions about how heat was transferred from the gas cap to the meteor and using kinetic theory, Lindemann and Dobson derived expressions for pa, the density of the atmosphere at the height of appearance, and pd, the density at the height of disappearance of the meteor. The equations are reproduced here to emphasize the large number of quantities involved, uncertainties in which could cause errors in the derived atmospheric densities.
\[ \rho_a = \frac{16}{3} \cdot \frac{\rho_m s T_2 r \cos X}{kv^2} \cdot \frac {g M_0}{RT_0} \]and
\[ \rho_d = \frac{24r}{V_1-V_2} \cdot \frac{\ell\Delta h}{vL} \cdot \rho_m \cdot \frac{gM_0}{RT_0} \]where
pm = density of the meteor
s = specific heat of the meteoric material
T2 = temperature of the surface of the meteor
r = radius of the meteor
X = angle of the meteor path to the vertical
g = acceleration of gravity
Mo = molecular weight of the air
k = (V1 - V2)/3v = calculated efficiency factor of heating
V1 = velocity of the compressed gas molecules in front of the meteor
V2 = velocity of the gas molecules at the temperature of the meteoric surface
ℓ = latent heat of vaporization of meteoric material
v = velocity of the meteor, assumed constant
R = universal gas constant
To = temperature of the atmosphere, assumed isothermal throughout the range of consideration
L = total length of the meteor trail
Δh = projection of L on the vertical.20
From the apparent brightness of the meteor the rate at which energy was being emitted could be calculated, which multiplied by the time of visibility gave the total amount of energy radiated. Setting this equal to the kinetic energy ½mv2 yielded the mass m of the meteor. If one then assumed that the meteor was iron and essentially spherical, one got from the expression
\[ \text{mass = density times volume} \] \[ m = P_m(4\pi r^3/3) \]which gave the radius r. The other quantities in the expressions for the atmospheric density could be either measured directly or estimated from plausible assumptions, thereby giving densities at two altitudes, that of appearance and that of disappearance.
The chain of reasoning was lengthy, with many assumptions. The results obtained by the investigators immediately put some of the assumptions into question. For example, the air densities obtained proved three times too high to correspond to an isothermal atmosphere at the stratospheric temperature of 220 K, requiring instead temperatures around 300 K. Between the stratosphere and the E region of the ionosphere, then, there had to be a significant variation in temperature. Moreover, other observations indicated that it was not even likely that the temperature would be constant in the E region. Experiments with the anomalous propagation of sound mentioned earlier showed that atmospheric temperatures rose markedly between 30 and 55 km to between 336 K and 350 K at the latter altitude. Noctilucent clouds, on the other hand, strongly suggested very low temperatures at 80 km.21 The conclusion was forced, then, that the atmosphere was not isothermal, having temperatures which rose sharply above the stratosphere to somewhere at or above 55 km, fell again to very low values around 80 km, and then rose once more between 80 and 100 km.
Disagreements also arose over how the meteors became incandescent. One investigator objected to the idea of a gas cap, preferring to assume that the meteor was heated by direct impact with the air molecules.22 In the early 1940s Fred Whipple obtained very accurate photographic records of meteor trails from which he could deduce decelerations. He developed an elaborate theory of how the properties of the upper atmospheric gases, the deceleration of the observed meteor and its heating to vaporization and incandescence, and its physical properties were all interrelated. Then, making some suppositions about properties of the incoming meteors and measuring deceleration and luminosity from the photographs, Whipple finally deduced the densities of the atmosphere along the trail.23 Again there were assumptions and corresponding uncertainties in the results.
For the ionosphericist the theoretical maze was even more complicated. The prober’s principal tool was the radio wave. A signal sent into the ionosphere would be bent by the ionized medium, and if the charge density were great enough would be reflected downward again. For a simple layer in which the strata of equal ionization were horizontal, the condition for total reflection of a signal propagated vertically was:
\[ (4\pi N^0_e e^2)mp^2 = 1 \]where
N0e = value of the electron density at the point of reflection
e = the electronic charge
m = mass of the electron
p = angular frequency of the radio signal.24
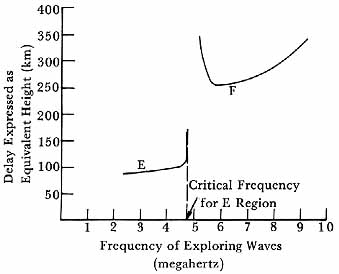
Thus, a radio signal of low enough frequency sent into the ionosphere would continue upward until it reached a level at which the electron density was great enough to satisfy equation (7). At that point the wave would be reflected, returning to the ground after a delay corresponding to its flight along the upward and downward paths. As the wave frequency was increased, the wave would penetrate farther into the layer before being reflected, and the delay in the ionosphere would be increased. If the layer had a maximum electron density, when the signal frequency exceeded the value (called the critical frequency) for which that maximum charge density would produce total reflection, then the wave passed through the layer and no return was observed at the ground.
By sweeping the signal frequency from low values to higher ones, one could generate a record of signal returns which could be displayed as shown in figure 5, curve E. The critical frequency could be read from the figure, from which the charge density at the point of reflection could then be calculated, using equation (7). With a little additional calculation, the height of the point of reflection could also be estimated, showing where the reflecting layer existed.
If, in the charge density, other maxima lay above and exceeded the initial maximum, then as the wave frequencies were increased new reflections would be observed, corresponding to the higher-altitude, more intensely ionized layers, as shown in curve F of figure 5. From the critical frequencies for these higher layers, estimates could be derived for the charge densities and heights of the upper layers.
By using an appropriate theory like that of Chapman concerning the formation of ionized layers by solar radiations (fig. 3), one could then estimate charge densities above and below the maxima obtained from the radio propagation measurements, and thus construct a continuous curve of charge densities versus altitude.
The concept was simple, but enormous complications entered when all the pertinent factors were considered. First, the ionosphere was by no means as simple as assumed in the foregoing example, and at times the propagation measurements indicated gross inhomogeneities. Moreover, one had to take into account the earth’s magnetic field, collision frequencies among the particles in the ionosphere, and the fact that the ionization consisted not only of electrons but also of both positive and negative ions. The earth’s magnetic field produced double refraction of the radio signals used to probe the ionosphere, splitting the signal into what were called ordinary and extraordinary rays, which followed different paths, had different points of reflection and different delay times, and were differently polarized-that is, the electric vectors of the two rays vibrated in different planes. When there were several ionospheric layers to deal with, and particularly under disturbed conditions, the problem of identifying properly the various return signals could become next to impossible. In addition, when the signal had to traverse a region in which the collision frequencies were high, as in a strong D region during times of high solar activity, the signal could be greatly attenuated or even blanked out. Not knowing the ions in the ionosphere simply added to the complication.
The mathematical expression of how all these factors affected the propagation of signals through the ionosphere was far more complicated than the simple expression of equation (7), and applying it to the determination of charge densities in the ionosphere put great demands on ingenuity and insight.25
These two examples of how investigators restricted to working with observations obtained at or near the ground had to wrest the information they sought from long chains of supposition and theoretical reasoning illustrate the sort of opportunity that befell the rocket researchers, who expected to make direct measurements in situ. Since much, even most, of what went on in the upper atmosphere was caused directly or indirectly by energy from the sun, a most important contribution the rocket sounder could make was to measure the solar spectrum both outside the appreciable atmosphere and as affected by altitude within the atmosphere. Knowing the former would let the theorist know what wavelengths and intensities were generating ionization, various photochemical reactions, and ultimately heating in the atmosphere. Knowing the latter would immediately tell where the different wavelengths were having their effect. The importance Mitra put on this vital information is seen in his assertion that “the greatest obstacle in the study of the upper atmosphere, is undoubtedly the lack of our direct and precise knowledge of the energy distribution in the near and extreme ultraviolet radiation of the sun. For, conditions in the high atmosphere are almost entirely controlled by the sun.”26
Many data the sounding rocket could obtain apparently could be obtained in no other way. In addition, many quantities that could be estimated from ground-based studies contained serious uncertainties which could be removed or lessened by rocket measurements. These circumstances made it possible for a number of young rocket experimenters in short order to compete respectably in upper-atmosphere research against much more knowledgeable scientists of many years’ experience. The ways in which newcomers could contribute may be illustrated by listing some of the problems that in the mid-1940s still awaited solution.27
Diurnal, seasonal, and other temporal variations in atmospheric pressure, temperature, and density were needed.
A correct description of atmospheric composition at all altitudes would be invaluable. One could determine the distribution of ozone in the upper stratosphere and middle atmosphere and find the level at which most of the ozone was formed. Knowing the composition would also allow one to know definitely to what altitudes the atmosphere was completely or nearly completely mixed, and at what altitudes diffusive separation played an important role. In particular one would want to know where oxygen began to dissociate into atomic form and at what altitude the dissociation had become complete, and whether at some altitudes nitrogen also dissociated. At what level would lighter gases like helium become an appreciable or even dominant component of the air?
With respect to the ionosphere radio sounding could not determine the ionization intensities in a region lying above one of higher charge density. One had to rely on theory to try to fill in the missing information. But in situ measurements might remove this lack. Moreover, if the precise nature and concentrations of both the positive and negative ions could be determined, a better understanding could be developed of how the balance between those agents creating the ionosphere and those tending to destroy it was established. One would then be in a better position to determine the specific causes of the temporal and geographic variations in the various ionospheric layers.
There was little doubt that excitation, dissociation, and ionization of atmospheric constituents, as well as various energy transfer and recombination processes, were responsible for the night sky radiations; but there were various possibilities among which to choose. Moreover, there were gross uncertainties in the altitudes from which many of the radiations were thought to arise. Again in situ measurements should help to resolve the difficulties, not only by pinning down altitudes, but also by providing additional insight into the recombination coefficients and other fundamental parameters involved.
As for magnetic field effects, a prime target would be to locate the electric currents that were responsible. One would hope, too, to be able to detect and identify the particles that caused the auroras.
With regard to cosmic rays, the precise composition of the primary radiation needed to be determined; for this purpose, measurements in outer space well above the atmosphere of the earth should be useful. Additional information on the effect of the earth’s magnetic field upon the cosmic rays would be interesting, but more fundamental would be data on whether the radiation was isotropic or anisotropic in free space. An intriguing question was how many of the cosmic rays coming to the earth were from the sun and how many were from outside the solar system.
THE HARVEST
Such were the problems to which the rocket experimenters addressed themselves. Once started, the results of their research flowed in a steady stream into the literature, contributing to a growing understanding of upper atmospheric phenomena. A concise summary of some of the more important results from the first dozen years of high-altitude rocket sounding appears in the author’s book Sounding Rockets.28 A deeper, more detailed insight into what had been achieved may be had from volume 12 of the Annals of the International Geophysical Year.29 The following brief review is derived from these and other sources.
It is not surprising that the first questions taken up by the rocket experimenters were those considered the most significant by the ground-based researchers. Naval Research Laboratory investigators built spectrographs and sent them aloft to photograph the solar spectrum at high altitude. On 10 October 1946 Richard Tousey and his colleagues obtained the first photographs of solar spectra from above the ozonosphere.30 This event marked the beginning of many years of intensive research on the structure and energy content of the solar spectrum in both the near and far ultraviolet and eventually in the x-ray region, using a variety of techniques including spectrographs, photon counters, and photosensitive phosphors.31 Experimenters at the Applied Physics Laboratory of the Johns Hopkins University quickly followed up the NRL achievement with spectrographic experiments of their own, obtaining highly detailed spectrograms.32 In March 1947 the Naval Research Laboratory workers obtained additional spectra at various altitudes reaching to 75 km, and in June 1949 more spectrograms were recorded.33 In the years that followed, both University of Colorado and Navy workers developed pointing devices to keep rocket-borne spectrographs aimed at the sun, and with these obtained more detail and continually extended the spectra to shorter and shorter wavelengths. Using the pointing control, the group at the University of Colorado in 1952 flew a spectrograph to about 85 km. In addition to the by now familiar ultraviolet spectrum from 2800 Å to about 2000 Å, there was a strong emission line at 1216 Å. This was quickly identified with the Lyman alpha line of the neutral hydrogen atom.34 Between 1952 and 1955 both the Naval Research Laboratory and Air Force groups confirmed the presence of other emission lines between 1000 Å and 2000 Å. In 1958 the University of Colorado team used a specially designed spectrograph to photograph the solar spectrum from 3000 A all the way to 84 Å in the extreme ultraviolet.35 About 130 emission lines were measured and their intensities roughly estimated. The resonance line of ionized helium at 304 Å was found to be very strong. In the years following, the Colorado workers, those at the Naval Research Laboratory, and a group at the Air Force Cambridge Research Center in Massachusetts contributed much detail on the solar spectrum in the far ultraviolet.
As had been anticipated, the ultraviolet spectrum of the sun, which proved to be very complex, did not correspond to a simple black body radiating at a 6000 K temperature as in the visible part of the spectrum. This finding was dramatically shown in a comparison of actual intensities obtained by NRL on 7 March 1947 with the 6000 K blackbody curve, shown in figure 6.
On 5 August 1948 in an Aerobee rocket flight to 96 km, T. R. Burnight detected what appeared to be x-rays in the upper atmosphere. Burnight did not follow up on his discovery, however, and it was left to others to pursue the subject.36
These data on the solar spectrum below the atmospheric cutoff at about 2800A supplied theorists with much of the missing information to explain how and where the sun’s radiation produced different atmospheric layers. The workers at the Naval Research Laboratory and the Applied Physics Laboratory used observations on the change in solar ultraviolet intensities with altitude to determine the distribution of ozone in the upper atmosphere.37 It was established that the level of maximum ozone production lay in the vicinity of 50 km, hence that the higher concentrations of ozone a lower altitudes had to be due to atmospheric circulations.
Solar ultraviolet could be tied with confidence to the E region of the ionosphere. The intense Lyman alpha radiation of the neutral hydrogen atom penetrated to 70 km and influenced the lower E region and upper D region of the ionosphere. But x-rays in the vicinity of 2 Å penetrated deep into the D region and were far more efficient in producing ionization in the D layer than was hydrogen Lyman alpha.
Atmospheric structure-that is, the variation of pressure, temperature, and density with altitude-also received the early attention of the rocket experimenters.38 Almost every flight carried gauges to measure these fundamental parameters. Signal Corps and University of Michigan groups adapted anomalous sound propagation techniques to the rocket by sending explosive grenades aloft to be set off at high altitude; the sound waves could be used to determine both air temperatures and winds up to 60 km or higher.39 Those measuring x-ray intensities used the observed absorption of x-rays in the ionosphere to estimate air densities there.40 As a result of many rocket observations, in the early 1950s the Rocket and Satellite Research Panel was able to issue an improved estimate of upper-atmospheric structure for use by geophysicists.41 By the time Sputnik went into orbit, the groundwork had been laid to describe the structure through the F region of the ionosphere and to give a considerable amount of information about both geographical and temporal variations of these quantities.42
The ionosphere was also receiving immediate attention in the sounding rocket program. Among the early experimenters, J. Carl Seddon undertook to adapt the propagation techniques of the ground-based probers to the rocket. He used the influence of the ionosphere on radio signals from the flying rocket to deduce charge densities existing in the atmosphere.
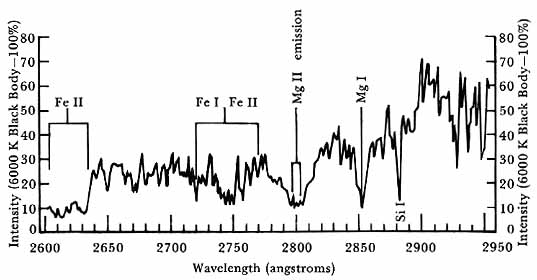
Solar spectrum. Solar intensities above the ozonosphere at White Sands, New Mexico, Spectrum of 7 March 1947, replotted on a linear intensity scale relative to the intensity of a black body at 6000 K. Durand et al, Astrophysical Journal 109 (1949): 1-16. Illustration courtesy of the Astrophysical Journal, published by the University of Chicago Press, copyright 1949, The American Astronomical Society. All rights reserved.
The phase speed c, wavelength X λ , and frequency f of a steady-state radio signal satisfy the equation
\[ c = Xf \]while the relation between c and c0, the phase velocity in free space, is
\[ c = c_0 /n \]where n is called the index of refraction of the medium in which c is the phase velocity. If the signal source is in motion relative to the observer, a shift in frequency, the well known doppler shift, results:
\[ \begin{aligned} \Delta f = -f(v/c) \\ = -fnv/c_0 \end{aligned} \]The original transmitted frequency could be carefully fixed in an experiment, Δf and v could be measured, and c0 would be a known constant. Hence n could be calculated. Since n depended on the electron and ion concentrations, their collision frequencies, and the strength and direction of the magnetic field, one could thus get an equation relating, the very quantities to be determined.43 Seddon arranged his experiment so as to get several such equations, which could be solved simultaneously to give electron densities as a function of height, and sometimes some of the other quantities such as collision frequencies.
Although transmitting the probing signal from the flying rocket was supposed to reduce the complexity, many of the difficulties experienced by the ground-based probers remained. Inhomogeneities in the ionosphere, multiple reflections of the propagated wave, splitting of the signal into ordinary and extraordinary rays, and not knowing the identities of the ambient ions made the reduction and interpretation of the data a challenge. Nevertheless, Seddon was able to improve upon electron density curves obtained from the ground and to furnish some information about the lowdensity regions that had been hidden from the probing of the ground-based investigators. Figure 7 shows a curve of electron density changing with altitude, drawn by John E. Jackson from a composite of NRL data and measurements by other groups.
Other experimenters preferred to avoid the problems inherent in propagation experiments by using various kinds of ionization gauges. Even though the rocket introduced complications of its own, such as exuding gases carried from the ground and distorting the ambient electric field, such gauge measurements were felt to be more “direct" than those obtained from the propagation experiments. Both techniques made their contributions, with the result that ground-based experimenters were provided with a standard, one might say, against which they could calibrate the methods of deducing results from their cheaper, more widespread observations.
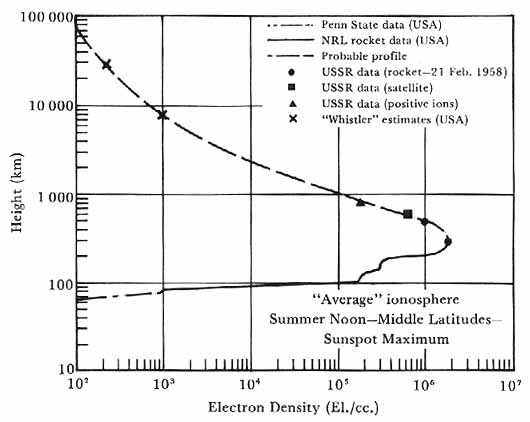
Ionospheric charge densities Summary as of August 1958 of ionospheric data corresponding to summer noon, middle latitudes, and sunspot maximum. Courtesy of J. E. Jackson, CSAGI meeting, Moscow, 1958.
Since the vexing question of composition continually entered into discussions of the upper atmosphere, particularly special regions like the ionosphere and the exosphere or fringe region at the top of the atmosphere, investigators soon tackled the problem of identifying atmospheric constituents as a function of altitude. At altitudes up to the bottom of the E region, workers from the University of Michigan tried sampling the air by opening evacuated glass vials or steel bottles in the upper atmosphere and immediately resealing them to lock in the sample before the rocket descended It was tricky, because one had to ensure that the bottles weren’t sampling gases carried by the rocket itself and also that the sampling procedure was not somehow altering the composition of the sample. While these experiments provided some hints of diffusive separation of helium over limited ranges above the stratosphere, by and large they confirmed that the atmosphere was thoroughly mixed, up to the E region.44
The most powerful technique to be brought to bear upon the problem of atmospheric composition was that of the mass spectrometer.45 This device separates out the atmospheric particles in accordance with their molecular masses-or, more properly, in accordance with the ratios of these to their charges in the ionized state in which they are fed to the spectrometer’s analyzer. While there can be some ambiguity, one can feel considerable confidence in the identifications achieved. With such an instrument John W. Townsend, Jr., and his colleagues at the Naval Research Laboratory produced a considerable amount of data on upper atmospheric composition above White Sands, New Mexico, and over Churchill, Canada.46 They confirmed that there was little diffusive separation below 100 km; but above 120 km separation processes, at least as indicated by the separation of argon relative to nitrogen, became quite effective. The changeover from molecular oxygen to atomic oxygen appeared to be slower than had been supposed. Neutral nitric oxide, NO, was shown to be a negligible constituent of the E region and above, since its presence would have been apparent in a pronounced absorption in the ultraviolet. No such absorption was observed in rocket solar spectrograms. On the other hand, NO+ turned out to be a major positive ion in the E region of the ionosphere. In northern latitudes, during the daytime above Fort Churchill, as altitude increased from 100 to 150 to 200 km the relative abundances of positive ions changed from (O+2, NO+) to (NO+, O+2, O+) to (O+, NO+, O+2). In the United States above White Sands the results were similar except that the nitric oxide ion NO+ was the predominant ion in the E region. In all cases O+ was the predominant positive ion above 250 km, while according to Soviet data N+ was never more than about 7% of the O+ for altitudes up to more than 800 km. On several flights the negative nitrogen dioxide NO2- was detected in the E region.
Some of the uncertainties concerning the heights of emission of the night airglow were removed by rocket experiments.47 The atomic oxygen green line at 5577 Å was found to have its maximum at about 95 km, to show a sharp lower cutoff at 90 km, and to trail off at 120 km on the upper side. The sodium D lines (5890 Å - 5896 Å) came primarily from the region between 75 and 100 km, peaking at about 90 km. The red oxygen lines (6300 A - 6364 Å) came from above 163 km, while the 6257 Å Meinel hydroxyl, OH, band was emitted in the region from 50 to 100 km.
Although some measurements were made of the earth’s magnetic field at high altitude and of associated current flows,48 this aspect of the high atmosphere received less attention during the first decade of rocket sounding than it would later when satellites became available. Cosmic rays were, however, a matter of intense interest to a few researchers. Of the many aspects of this fascinating subject to pursue, two topics in particular stood out: (1) What was the cosmic ray intensity above the atmosphere? (2) What was the composition of the cosmic rays? James A. Van Allen tackled these questions in a rather straightforward way. By sending a single geiger counter into the upper atmosphere, he was able to trace out a counting rate curve that rose to a Pfotzer maximum at a height of about 19 km, above which the remaining atmosphere corresponded to about 56 g/cm2 of material (fig. 8).49 With increasing altitude beyond that level the counting rate declined until it leveled off at a constant rate at and above 55 km. After several flights Van Allen was able to estimate the vertical intensity of cosmic rays at high altitude above White Sands to be 0.077 ± 0.005 particles per sec-cm2-ster,* close to the value that workers at the Naval Research Laboratory obtained. With rather poor statistics the rocket experimenters estimated that the primary cosmic rays consisted of protons and alpha particles in the ratio of about 5 to 1, with less than one percent heavier nuclei.50 These figures differed somewhat from better measures being obtained from balloon observations.
The energy spectrum of the cosmic rays had suggested a distinct lower bound for the cosmic ray particles. Van Allen began to investigate the lower energy end of the cosmic ray spectrum. He sent counters aloft at latitudes ranging from the geomagnetic equator to the polar regions. During these investigations, Van Allen noted a pronounced increase in the numbers of soft radiation particles encountered above the stratosphere in the auroral zone, particles that were not found at either lower or higher latitudes.51
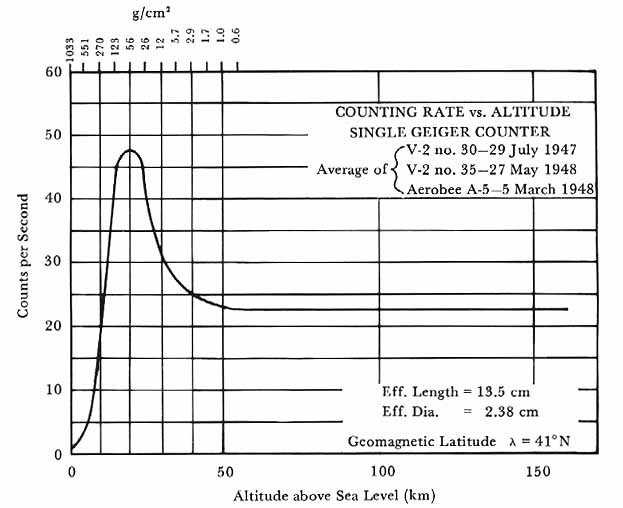
Cosmic ray flux. Smoothed composite curve of Applied Physics Laboratory single-counter counting rates above White Sands, New Mexico, geomagnetic latitude 41°N. Gangnes, Jenkins, and Van Allen in Physical Review 75 (1949). 57-69, courtesy of J. A. Van Allen and Physical Review
SIGNIFICANCE
The foregoing nontechnical description is merely illustrative. A review of a length appropriate to this book cannot cover in detail 12 years of work by hundreds of scientists. Nor can the description convey to the reader the many subtleties and innumerable interrelationships with which both experimenters and theorists concerned themselves. Nevertheless, brief though it is, the summary shows how the rocket sounding work contributed to atmospheric and cosmic ray research.
The new tool, the high-altitude research rocket, had indeed made it possible to obtain data hitherto unobtainable and to solve problems hitherto intractable-as anticipated. The rocket results enabled ground-based observers to improve their techniques and to obtain better results from their measurements-that is, to calibrate their experiments. Whereas at the start some had expressed grave doubts as to the wisdom of using rockets for high-altitude research, a decade later the importance of the sounding rocket to the field was universally recognized. It is natural, then, to ask whether sounding rockets had revolutionized the field of upper atmospheric research.
In the excitement of new discoveries amid a continuing flow of important data from a long list of topics-solar physics; atmospheric pressure temperature, density, composition, and winds; the ionosphere; magnetic fields; the airglow; the auroras; and cosmic rays-the rocket experimenters liked to think and speak of their work as revolutionizing the field. But it is clear in retrospect that the first decade of high-altitude rocket research was normal science, not revolution. Put otherwise, the results from those year of research elaborated and expanded upon the already accepted paradigm but did not force any fundamental changes in it.
Were one to compose a schematic diagram of the upper atmosphere based on what was known immediately following the launching of Sputnik, the picture would probably look much like the drawing of figure 9. A comparison with figure 1 drawn from information set forth in Mitra’s book of 10 years earlier shows a striking similarity in overall concepts. In both, the atmosphere is visualized as consisting of a number of characteristic layers-troposphere, stratosphere, ozonosphere, ionosphere, and exosphere-at essentially the same altitude levels. In both, temperatures vary markedly with altitude, and these variations are associated with the different atmospheric layers. Solar radiations are considered to be the cause of photochemical processes going on in the atmosphere, affecting composition, giving rise to the night airglow, and forming the ozonosphere and ionosphere. Heating of different levels in the atmosphere is ascribed to incoming solar energy, which in a series of stages ultimately degenerates into heat. There is little doubt that the auroras are caused by charged particles from the sun, and that in some way such particles are also responsible for changes in the earth’s magnetic field during magnetic storms.
Clearly the two paradigms, before and after, are essentially the same. The expert will, of course, see a new richness of detail in the later picture, but nothing that the earlier paradigm could not accommodate once the facts were known. Thus, space science’s first decade, the sounding rocket period, must be characterized as extremely fruitful normal science. Nevertheless, in that early harvest were the elements of some remarkable discoveries.
The soft radiation that Van Allen had detected in the auroral regions presaged the discovery of a largely unsuspected aspect of the earth’s environment. Following up his interest in these soft radiations, Van Allen instrumented the first American satellite, Explorer 1 , with counters to probe further the incoming cosmic rays. Unexpectedly high radiation intensities were found above the atmosphere and, after additional measurements in Explorer 3, Van Allen on 1 May 1958 announced the discovery of a belt of radiation surrounding the earth, which at once became known as the Van Allen Radiation Belt.52 The discovery set in motion a long chain of investigations that in the course of the next several years forced a revision of the picture scientists had developed of how the sun’s particle radiations affect the earth’s atmosphere. The new features of the geophysical paradigm will be presented in chapter 11.
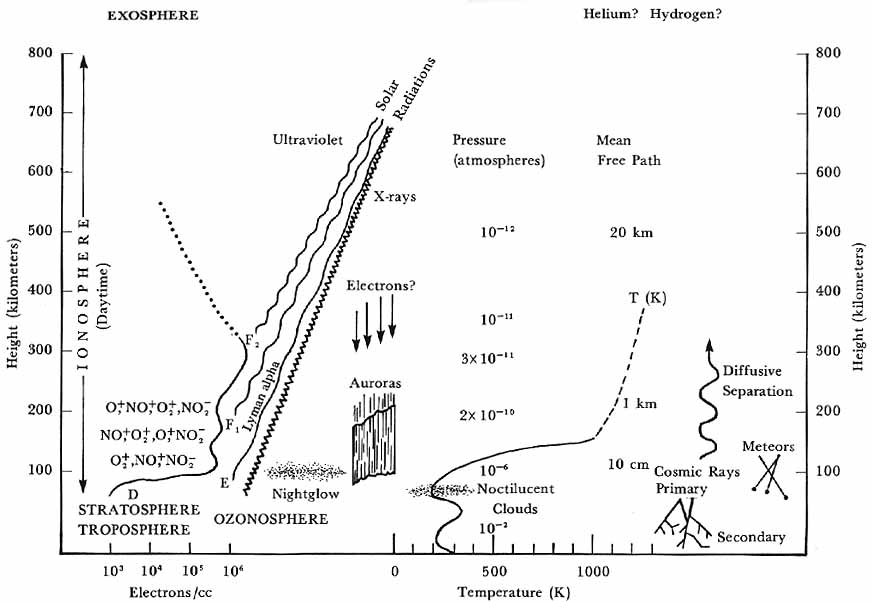
Upper atmosphere as visualized in 1958. The general features are similar to those of figure 1, corresponding to the mid-1940s, but there is much more detail.
The second discovery came from the rocket investigations of the sun’s short-wavelength spectrum. The discovery that x-rays were an important variable in the solar spectrum suggested that x-rays might also be important in other stars and celestial objects, which later proved to be correct.53 When the experimenters in the Naval Research Laboratory group turned their ultraviolet and x-ray detectors toward the stars, they initiated a new field of rocket astronomy, which will be described more fully in chapter 20.
In the meantime, the early harvest from rocket sounding of the upper atmosphere was convincing evidence of the rich returns that could be expected from a program of scientific research in space. In this aspect of space, at least, the United States could consider itself fully competitive with any rival.
- ster, short for steradian, a common measure of solid angle.
Source Notes
- Homer E. Newell, Jr., High Altitude Rocket Research (New York: Academic Press, 1953), pp. 111-42; the Rocket Panel, “Pressures, Densities, and Temperatures in the Upper Atmosphere,” Physical Review 88 (Dec. 1952): 1027-32.X
- B. Haurwitz, “The Physical State of the Upper Atmosphere,” reprinted from Journal of the Royal Astronomical Society of Canada, Oct. 1936-Feb. 1937 (Toronto: Press, 1937. with addition, 1941).X
- T. H. Johnson. “Cosmic Ray Intensity and Geomagnetic Effects,” Reviews of Modern Physics 10 (Oct. 1938): 193-244.X
- Fred L. Whipple, “Meteors and the Earth’s Upper Atmosphere,” Reviews of Modern Physics 15 (Oct. 1943): 246-64.X
- S. K. Mitra, The Upper Atmosphere (Calcutta: Royal Asiatic Society of Bengal, 1947). 519.X
- Ibid., pp. 5-8.X
- Ibid., p. 511.X
- B. Stewart, in Encyclopaedia Britannica, 9th ed., 16 (1882): 181.X
- A. E. Kennelly,” On the Elevation of the Electrically-Conducting Strata of the Earth’s Atmosphere", Electric World and Engineering 39 (Mar. 1902):,173; O. Heaviside, “Telegraphy-Theory.” Encyclopaedia Britannica, 10th ed., 33 (1902): 213-18.X
- E. V. Appleton and M. A. F. Barnett, “Local Reflection of Wireless Waves from the Upper Atmosphere, Nature 115 (March 1925): 333-34; idem, “On Some Direct Evidence for Downward Atmospheric Reflection of Electric Rays,” Proceedings of the Royal Society, A 109 (1925): 621-41.X
- G. Breit and M. A. Tuve, “A Test of the Existence of the Conducting Layer,” Physical Review 28 (Sept. 1926): 554-75.X
- Mitra, Upper Atmosphere, chap. 6.X
- Ibid., pp. 257-61.X
- Ibid., p. 512.X
- Ibid., pp. 328-30.X
- See, for example, C. Stormer, “Twenty-five Years Work on the Polar Aurora,” Terrestrial Magnetism and Atmospheric Electricity 35 (1930): 193-208.X
- Newell, High Attitude Rocket Research, pp. 237-45.X
- See also I. S. Bowen, R. A. Millikan, and H. V. Neher, “New Light on the Nature and Origin of the Incoming Cosmic Rays,” Physical Review 53 (June 1938): 855-61.X
- M. Schein, W. P. Jesse, and E. O. Wollan, “The Nature of the Primary Cosmic Radiation and the Origin of the Mesotron,” Physical Review 59 (Apr. 1941): 615.X
- Mitra, Upper Atmosphere, pp. 77-87.X
- Haurwitz, Upper Atmosphere, p. 79: p. 8.X
- Mitra, Upper Atmosphere, p. 87.X
- Whipple, “Meteors and the Earth’s Upper Atmosphere.”X
- Mitra, Upper Atmosphere, p. 146.X
- Ibid., p. 151.X
- Ibid., p. 518.X
- Ibid., pp. 515-19.X
- Homer E. Newell, Jr., Sounding Rockets (New York: McGraw-Hill fill Book Co., 1959), pp. 37-43.X
- Homer E. Newell Jr., and Leonard N. Cornier, eds., First Results of IGY Rocket and Satellite Research, vol. 12, pts. 1 and 2, in Annals of the International Geophysical Year (London: Pergamon Press. 1960).X
- W.A. Baum, F. S. Johnson, J. J. Oberly, C. C. Rockwood, C. V. Strain. and R. Tousey, “Solar Ultraviolet Spectrum to 88 Kilometers.” Physical Review 70 (Nov. 1946): 781-82.X
- Herbert Friedman, “The Sun’s Ionizing Radiations,” chapter in Physics of the Upper Atmosphere, ed. J. A. Ratcliffe (New York: Academic Press, 1 1960), pp. 133-218.X
- J. J. Hopfield and H. E. Clearman, Jr., “The Ultraviolet Spectrum of the Suit from V-2 Rockets,” Physical Review 73 (Apr. 1948): 877-84.X
- E. Durand, J. J. Oberly, and R. Tousey, “Analysis of the First Rocket Ultraviolet Spectra,” Astrophysical Journal 109 (Jan. 1949): 1-16.X
- W.A. Rense. “Intensity of Lyman-Alpha Line in the Solar Spectrum,” Physical Review 91 (15 July 1953): 299-302; idem, “Solar Ultraviolet Radiation and Its Effect on the Earth’s Upper Atmosphere,” in Advances in Space Research, ed.T. M. Tabanera et al. (London: Pergamon Press, 1964). pp. 275-76.X
- Rense, “Solar Ultraviolet and Its Effect Earth’s Upper Atmosphere,” pp. 278-79.X
- Newell, High Altitude Rocket Research, p. 161; Friedman, “The Sun’s Ionizing Radiations,” pp. 168-78.X
- F. S. Johnson, J. D. Purcell, and R. Tousey. “Measurements of the Vertical Distribution of Atmospheric Ozone from Rockets,” Journal of Geophysical Research 56 (Dec. 1951): 583-94; F. S. Johnson et al., “Direct Measurements of the Vertical Distribution of Atmospheric Ozone to 70 Kilometers Altitude,” Journal of Geophysical Research 57 (June 1952): 157-76: J. A. Van Allen and J.J. Hopfield, “Preliminary Report on Atmospheric Ozone Measurements from Rockets,” in septembre L’etude optique de 1’atmosphere terrestre., communications presentees au colloque international tenu à l’Institut d’Astrophysique de I’Universite de Liege les 3 et 4 septembre 1951 (Louvain: Imprimere Centerick, 1952), pp. 179-83.X
- Newell, High Altitude Rocket Research, pp. 111-42; idem, “The Upper Atmosphere Studied by Rockets and Satellites,” chapter in Ratcliffe, Physics of the Upper Atmosphere, pp. 74-101.X
- W. G. Stroud, W. Nordberg, W. R. Bandeen, F. L. Bartman, and P. Titus, “Rocket Grenade Measurements of Temperature and Winds in the Mesosphere over Churchill, Canada,” in Space Research Proceedings of the First International Space Science Symposium, Nice, 1960, ed. H. K. Kallmann-Bijl (Amsterdam: North-Holland Publishing Co., 1960), pp. 117-43.X
- Friedman, “The Sun’s Ionizing Radiations.” pp. 208-14.X
- The Rocket Panel, “Pressures, Densities, and Temperatures in the Upper Atmosphere.”X
- Newell, The Upper Atmosphere Studied by Rockets and Satellites,” pp. 74-97.X
- 43 Ibid., pp. 102-11.X
- Newell. High Altitude Rocket Research, pp. 203-08.X
- J. W. Townsend, Jr., “Radio frequency Mass Spectrometer for Upper Air Research,” Reviews of Scientific Instruments 23 (1952): 538-41.X
- Newell, “The Upper Atmosphere Studied by Rockets and Satellites,” pp. 112-18. E. B. Meadows and J. W. Townsend, Jr., “IGY Rocket Measurements of Arctic Atmospheric Composition Above 100 Km,” in Space Research, ed. Kallmann-Bijl, pp. 175-98. C. Y. Johnson, “Aeronomic Measurements,” in Advances in Space Research, ed. Tabanera et al., pp. 295-317.X
- Newell, “The Upper Atmosphere Studied by Rockets and Satellites,” pp. 119-20.X
- Ibid., pp. 108-11.X
- A. V. Gangnes, J. F. Jenkins, Jr., and J. A. Van Allen, “The Cosmic Ray Intensity above the Atmosphere,” Physical Review 75 (Jan. 1949): 57-69.X
- G. J. Perlow et al, “Rocket Determination of the Ionization Spectrum of Charged Cosmic Rays at α 41° N.” Physical Review 88 (Oct. 1952): 321-25.X
- L. H. Meredith, M. B. Gottlieb, and J. A. Van Allen, “Direct Detection of Soft Radiation Above 50 Kilometers in the Auroral Zone,” Physical Review 97 (1 Jan. 1955): 201; J. A. Van Allen, “Rocket Measurement of Soft Radiation,” in First Results of IGY Rocket and Satellite Research, ed. Newell and Cormier, pp. 616-50.X
- Paper presented by Van Allen at joint meeting of National Academy of Sciences and Physical Society on 1 May 1958. See also, J. A. Van Allen, G. H. Ludwig, E. C. Ray, and C. E. McIlwain, “The Observation of High Intensity Radiation by Satellites 1958 and , in First Results of IGY Rocket and Satellite Research, ed. Newell and Cormier, pp. 671-81.X
- J. E. Kupperian et al. “Far Ultraviolet Radiation in the Night Sky.” in First Results of IGY Rocket and Satellite Research , ed. Newell and Cormier, pp. 619-22; J. E. Kupperian et al., “Rocket Astronomy in the Far Ultraviolet.” ibid., pp. 622-26; Herbert Friedman et al., Space Astronomy: A New Era in the Making, a series of articles reprinted from Astronautics & Aeronautics 7 (Mar. and May 1969), pp. 34-75. X